Zinc Water Treatment and Wastewater Removal
Industrial wastewater is produced as a by-product of the manufacturing of commercial products. Certain industries and processes produce wastewater that contains high concentrations of heavy metals, including zinc, which are toxic to the environment. These metals need to be properly removed prior to discharge to a body of water or sanitary sewer.
Zinc is one of the most common metals found in the wastewater of facilities that perform plating, galvanizing, and other metal finishing processes. While a certain concentration of zinc can be safely present in water, elevated levels are toxic and must be removed.
Consult Treatment Solutions
Please fill out the form above to get in touch. We look forward to assisting your with your water treatment needs.
Consult Zinc Removal Solutions With ACS
Zinc is a common metal found in industrial wastewater, but often it is not the only contaminant present. Advanced Chemical Systems (ACS) provides customized chemical and equipment solutions to address multiple contaminants and meet increasingly stringent effluent requirements. Send us a sample today, and ACS will carefully diagnose your specific situation and design a customized solids separation and metal precipitation solution to meet your needs.
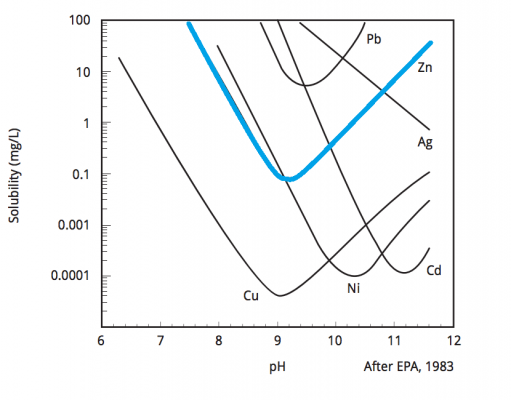
How to Remove Zinc From Water
Prior to discharge, excess zinc must be chemically and physically removed from the wastewater. This removal is achieved through a combination of specialized equipment and chemicals tailored to the specific metals present. As wastewater enters the treatment process, the zinc is in a stable, dissolved aqueous form.
The goal of zinc water treatment by hydroxide precipitation is to adjust the pH (hydroxide ion concentration) of the water so that the zinc will form an insoluble precipitate. Once the zinc precipitates and forms solids, it can then easily be removed. The water, now with low metal concentrations, can be discharged.
The exact treatment process will vary from one facility to the next, but in general, zinc can be precipitated to acceptable levels through the use of a blended coagulant and pH adjustment (9.5 ±1). This is a chemical process which renders the zinc insoluble, resulting in precipitated solids. A polymer is then added, which conglomerates the solids into a sludge that can be dewatered through equipment such as a filter press.
Further Treatment with Zinc Precipitant
In some cases, additional products such as a metal precipitant are incorporated into a treatment process to effectively treat more difficult wastes where chelating agents are present (citric acid, EDTA, NTA). The precipitant breaks the metal‐chelate compound into free metal and free chelating agent, followed by the standard treatment process. The chelating agent is discharged with the treated effluent.
